Key Takeaways
|
Two groundbreaking gene therapy treatments for sickle cell disease (SCD), offering potential cures, are on track for FDA approval with the expectation that both could be approved by December 2023. In April 2023, CRISPR Therapeutics (NASDAQ: CRSP) and Vertex Pharmaceuticals (NASDAQ: VRTX) submitted a Biologics License Application (BLA) for exa-cel (exagamglogene autotemcel), formerly known as CTX001. Exa-cel is intended to treat severe SCD and transfusion-dependent beta-thalassemia (TDT).
In the same month, Bluebird Bio (NASDAQ: BLUE) submitted a BLA for lovo-cel (lovotibeglogene autotemcel) for SCD patients 12 years and older with a history of vaso-occlusive events. Both submissions received FDA Priority Review status for SCD, indicating a regulatory decision within 6 months.
Exa-cel and lovo-cel
Exa-cel is a CRISPR-based gene editing approach to gene therapy, while lovo-cel is a lentivirus-based gene therapy. Exa-cel will be the first CRISPR-based therapy to be reviewed by the FDA — a major milestone for CRISPR gene editing, which was awarded the Nobel Prize in 2022. Both exa-cel and lovo-cel are potential one-time therapies that provide a functional cure for SCD by modifying the patient’s hematopoietic stem cells. Gene therapy approaches are particularly beneficial for those without a suitable bone marrow donor.
Current treatment options for SCD
Patients with SCD have limited treatment options and hematopoietic stem cell transplant (HSCT) is the only curative treatment for SCD that can restore red blood cell and hemoglobin function. HSCT is invasive, requires a suitable donor and is associated with immune rejection risk.
Other than HSCT, currently approved treatments for SCD address symptom management and prevention of complications. Pfizer’s (NYSE: PFE) Oxbryta is an oral medication for SCD approved in the U.S. and the EU that increases hemoglobin by inhibiting red blood cell sickling, improving red blood cell function, and reducing whole blood viscosity.
Novartis’s (NYSE: NVS) Adakveo is a P-selectin antibody that reduces the frequency of vaso-occlusive events and prevents SCD-related pain. Adakveo was approved in the U.S. in November 2019 and was granted conditional approval in the EU in October 2020. However, the EU revoked its authorization in August 2023 following an unsuccessful Phase 3 trial.
Shift toward gene therapy
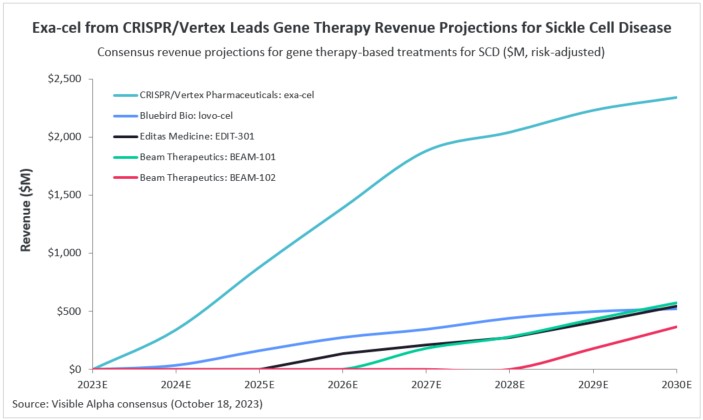
With the expected approval of gene therapy treatments this year, analysts expect a growing shift toward gene therapy as an effective treatment option for SCD. According to Visible Alpha consensus, Exa-cel (CRISPR and Vertex) for SCD is expected to generate $2.34 billion in risk-adjusted revenue by 2030. Analysts peg the probability of success for exa-cel at 77.8%. Bluebird Bio’s lovo-cel is expected to generate $520 million by 2030, with a probability of success of 78.3%.
Other gene therapy drugs in clinical trials for SCD include BEAM Therapeutics’ (NASDAQ: BEAM) BEAM-101 and BEAM-102, as well as Editas Medicine’s (NASDAQ: EDIT) EDIT-301. BEAM-101 is currently in Phase 2 trials, BEAM-102 is in the preclinical stage, and EDIT-301 is in Phase 1.
Figure 1: Gene therapy revenue projections for sickle cell disease (SCD)
By: Aarti Surendran
Reviewed By: Rahul Jasuja, PhD



