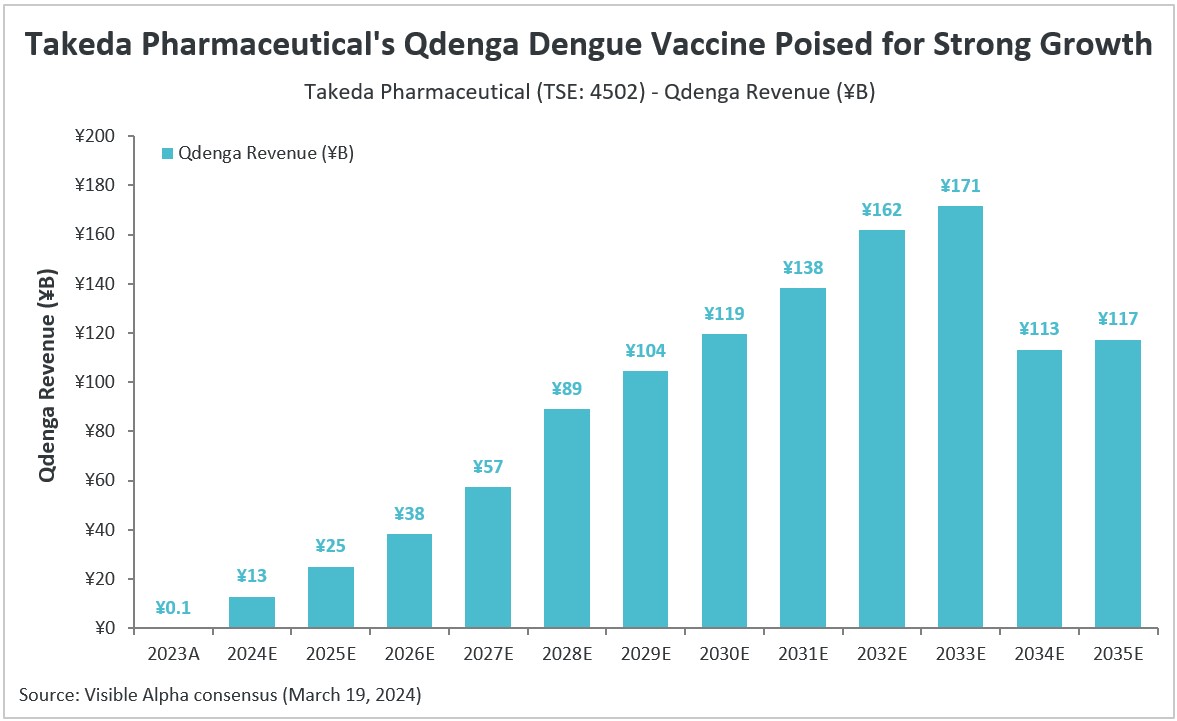
According to Visible Alpha consensus, Takeda Pharmaceutical’s (TSE: 4502) dengue vaccine Qdenga (TAK-003) is expected to generate ¥13 billion in revenue in 2024, and is anticipated to grow at a strong double-digit pace until 2032. Visible Alpha consensus revenue estimates show peak global sales of ¥171 billion for Qdenga by 2033. Sales are projected to dip starting in 2034 as Qdenga’s patent is set to expire on September 17, 2035.
The rise of Qdenga is timely, as two of Takeda’s leading drugs, Vyanase for attention deficit hyperactivity disorder (ADHD) and Azilva for hypertension, lost patent exclusivity in 2023, opening the door for generic competition.
About dengue fever and Qdenga
Dengue fever is caused by the dengue virus that is spread via the bite of an infected mosquito. According to the Centers for Disease Control and Prevention (CDC), a population of around 4 billion (half the world’s population) live in areas at risk for dengue virus infection. Each year, about 40,000 individuals die from serious dengue infection. An estimated 400 million get infected and about 100 million become ill from infection with dengue virus.
The Qdenga vaccine is based on a live-attenuated dengue serotype 2 virus that provides a genetic backbone for the four known dengue virus serotypes. It is designed to protect against all of the dengue virus serotypes. Qdenga aims to improve upon Sanofi’s Dengvaxia, the world’s first dengue virus vaccine. Following safety concerns, Dengvaxia’s use has been restricted to children aged 6 to 16 with evidence of prior dengue infection, specifically in endemic regions.
Qdenga not yet FDA-approved
First approved for use in Indonesia in 2022, Qdenga is currently approved in the U.K., Brazil, Argentina, Indonesia, Thailand, and Malaysia. Qdenga was approved in Europe in 2023. Approval by the FDA in the U.S. encountered obstacles. In November 2023, the FDA initiated a priority review for Qdenga, a process that typically takes less than six months, as opposed to the standard 10-month review. However, Takeda voluntarily withdrew its U.S. application because the FDA required additional data that Takeda had not captured in the Phase 3 trial. Takeda has not re-initiated the application with the FDA.
Reviewed by Rahul Jasuja, PhD