Among the next generation of GLP-1 based therapies currently in clinical development, Viking Therapeutics’ (NASDAQ: VKTX) VK2735, a dual GLP-1 and GIP agonist, is one of the most attractive weight-loss drug candidates based on recent Phase 2a trial data (Venture study) in obese patients. Viking is planning for VK2735 Phase 2b trials, and investors are paying close attention to any potential Big Pharma partnership or acquisition before Phase 3 trials. (See also our latest Visible Alpha GLP-1 Monitor for more on this family of drugs.)
Phase 2a obesity trial with VK2735
The Phase 2a study with VK2735 was a 13-week trial in obese patients with body mass index (BMI) ≥30, or ≥27 for patients with comorbidities. The study was a randomized, placebo-controlled study enrolling 176 patients. The primary endpoint of the study was percent change in body weight at 13-weeks versus placebo. The study arms included placebo, 2.5mg, 5mg, 10mg and 15mg VK2735 dose groups.
At the end of 13 weeks, the reduction in body weight was: 1.7% (placebo), 9.1% (2.5mg), 10.9% (5mg), 12.9% (10mg) and 14.7% (15mg). As depicted in Figure 1, the placebo-adjusted reduction in weight was 7.4% (2.5mg), 9.2% (5mg), 11.3% (10mg), and 13.1% (15mg). By any standard, these are impressive weight reductions within a 13-week timeline. At the end of the 13 weeks, there was no plateau observed, suggesting that weight loss would potentially continue with dosing. The study also achieved a significant secondary endpoint, demonstrating that 88% of patients experienced ≥10% weight loss.
Figure 1: Phase 2a Venture study with dual GLP-1/GIP agonist, VK2735, in obese patients
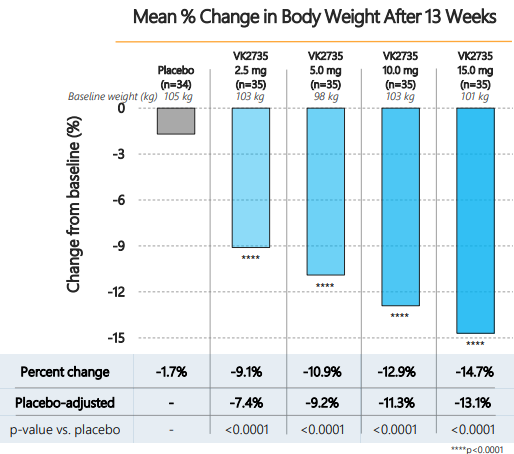
Source: VKTX presentation (May 2024). The Phase 2a showed as much as a 13.1% placebo adjusted reduction in body weight in the 15 mg dose group. The effect of VK2735 was dose dependent.
Treatment-emergent adverse events (TEAEs) that resulted in patients discontinuing the study were modest – no discontinuations in the placebo, 2.5mg, 5mg, or 10mg dose. There was only one patient discontinuation in the highest 15mg dose.
How does VK2735 compare with the competition?
While Phase 2a data with VK2735 is only available for 13 weeks, it appears promising, and longer-term data could lead to an even greater reduction in weight. As noted above, there was no plateau observed at 13 weeks, which implies that weight reduction may potentially continue with longer-term treatment. Figure 2 compares published data on GLP-1 based marketed drugs and drugs in clinical development with VK2735 Phase 2a 13-week data. Weight loss of 13.1% with VK2735 in 13 weeks is impressive. For a rough comparison, weight loss at 12 weeks with Eli Lilly’s (NYSE: LLY) tirzepatide (Zepbound) was 8%, as pointed out in the VKTX presentation (Figure 2, orange dotted line). Longer-term data with VK2735 is required for conclusive determination of comparative efficacy.
Figure 2: VK2735 13-week weight loss compared with published data for other GLP-1 based drugs on the market or in development
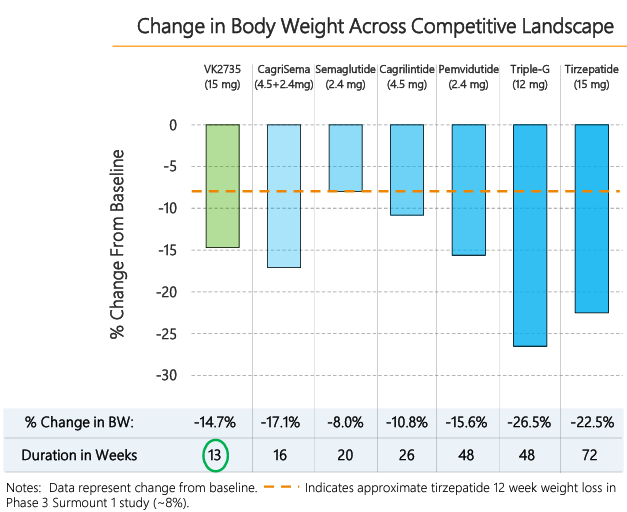
Source: VKTX presentation (May 2024). Novo Nordisk’s (NYSE: NVO) CagriSema (GLP-1 agonist + amylin receptor agonist) is in Phase 3 trials; NVO’s Semaglutide, Wegovy (GLP-1 agonist) is on the market; NVO’s Cagrilintide (amylin receptor agonist) is in Phase 2; Altimmune’s (NASDAQ: ALT) Pemvidutide (GLP-1/glucagon dual receptor agonist) has completed Phase 2 studies; LLY’s Retatrutide, also termed Triple-G (GLP-1, GIP, & glucagon receptors agonist) is in Phase 3 trials; and LLY’s Tirzepatide, Zepbound (GLP-1/GIP dual receptor agonist), is on the market.
Favorable pharmacokinetic data from Phase 1 study
Phase 1 single ascending dose studies showed that VK2735 has a favorable pharmacokinetic profile. Pharmacokinetic studies showed a T1/2 of 170 to 250 hours, making weekly (or even longer) dosing possible, and a Tmax of 75-90 hours indicates a slow onset of drug exposure that lends to the safety and efficacy of VK2735.
VK2735 consensus revenue projections
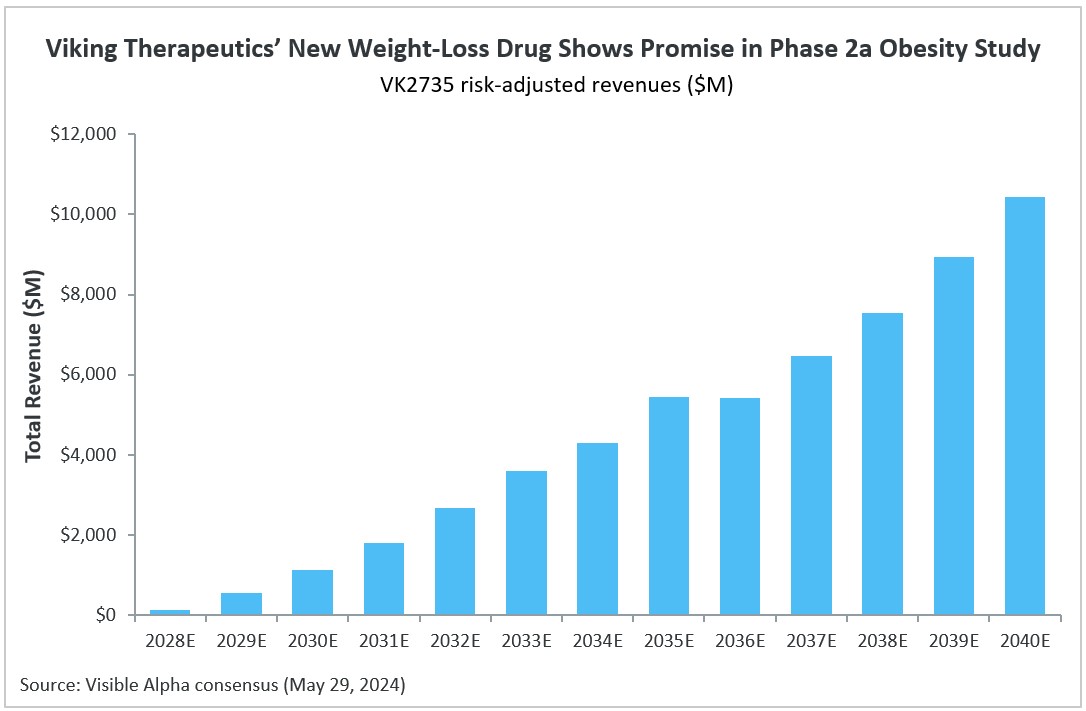
VK2735 is expected to be on the market in 2028. Visible Alpha consensus revenue projections (risk-adjusted) estimate VK2735 will reach $3.6 billion within 5 years of market approval in 2033. By the year 2040, VK2735 tops almost $10.5 billion in projected risk-adjusted revenues. Based on Visible Alpha consensus, the likelihood of VK2735 approval is 74.2%.
Figure 3: VK2735 risk-adjusted consensus revenue projections
Based on efficacy and safety data available to date in Phase 2a studies, VK2735 has the potential to become a lucrative asset in the obesity/weight management market. Viking plans to conduct a Phase 2b study in a larger patient population with 24-36 weeks dosing. This longer-term study within a larger patient population will add to the rigor of VK2735 data. In addition to subcutaneous VK2735, Viking is also developing an oral VK2735, currently in Phase 1 trials. Early data with oral VK2735 appears promising.