The FDA Advisory Committee evaluating Eli Lilly’s (NYSE: LLY) donanemab for Alzheimer’s disease unanimously recommended approval in patients with early Alzheimer’s disease with mild cognitive impairment and mild dementia. The FDA usually heeds the advice of its Advisory Committee but is not obligated to.
Donanemab Could Be on the Market Later in 2024
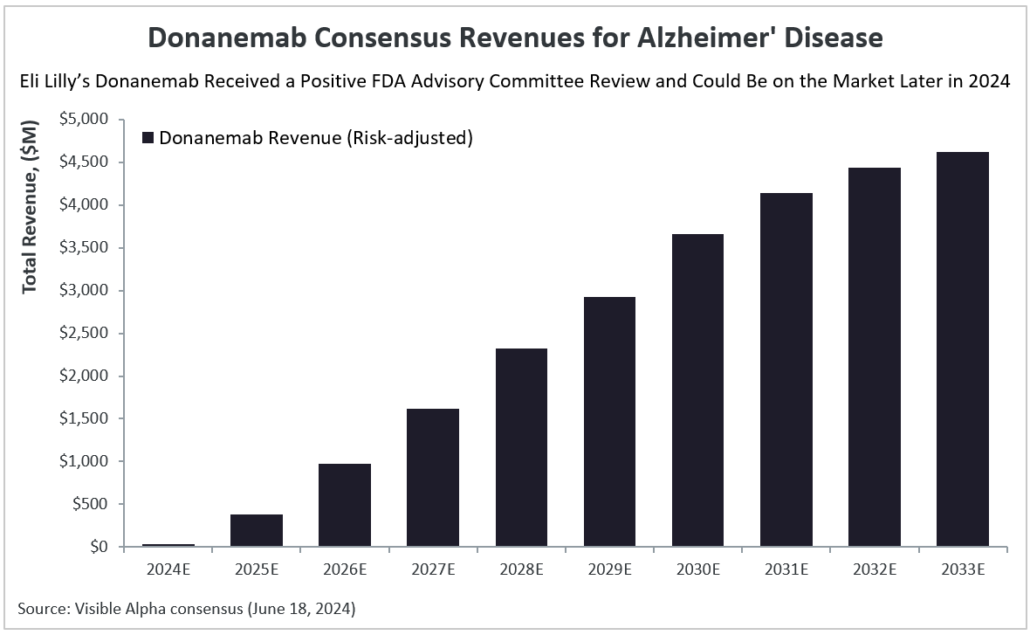
Donanemab is an amyloid β targeting antibody that follows in the footsteps of Biogen’s (NASDAQ: BIIB) & Eisai’s (TSE: 4523), Leqembi (lecanemab). Leqembi was the first amyloid β targeting antibody approved in July 2023. Visible Alpha consensus revenue estimates show donanemab reaching $4.6 billion in risk-adjusted revenues by 2033. The current Visible Alpha consensus probability of approval (POS) for donanemab is at 91.9%, however, the POS will likely increase as analysts update their models given the recent positive Advisory Committee review.
Donanemab FDA Advisory Committee Meeting
FDA’s Peripheral and Central Nervous System Drugs Advisory Committee met to discuss donanemab efficacy and safety on June 10, 2024. Donanemab was expected to be approved by the end of 2023, however regulatory delays related to safety evaluation prolonged the timeline. It is no surprise that the majority of the Advisory Committee meeting discussion was focused on the safety of donanemab, followed by evaluation of Tau protein levels in patients enrolled and the duration of donanemab treatment.
The Advisory Committee was voting on two questions:
- Does the available data show that donanemab is effective for the treatment of Alzheimer’s disease in the population enrolled in the clinical trials with mild cognitive impairment and mild dementia?
- Do the benefits outweigh the risks of donanemab in the treatment of Alzheimer’s disease in the population enrolled in the clinical trials with mild cognitive impairment and mild dementia?
On both voting questions, the Advisory Committee voted unanimously (11 to 0) in favor of efficacy and in favor of benefits outweighing risks.
The other notable areas of discussion at the Advisory Committee meeting included:
- Analysts believe that the FDA will likely approve donanemab with a broad label for early-stage Alzheimer’s disease, with close monitoring required for certain high-risk patient subgroups
- Even though the donanemab Phase 3 Trial (TRAILBLAZER-ALZ 2) stratified patients based on the levels of Tau protein (a predictive biomarker for Alzheimer’s disease progression), the Advisory Committee did not recommend Tau PET imaging for screening of patients for donanemab use. Tau protein PET imaging would have restricted the use of donanemab given the limited access to Tau PET imaging available to patients.
- Donanemab will likely be approved with a standard boxed warning for amyloid β targeting therapies – similar to Leqembi.
- The Advisory Committee was in agreement with the LLY approach to donanemab dosing, i.e. treating until amyloid β clearance as measured by an amyloid β PET scan. Therefore, dosing with donanemab will be stopped when the patient shows a negative amyloid PET scan.