X4 Pharmaceuticals (NASDAQ: XFOR) developed Xolremdi (mavorixafor), a CXCR4 chemokine receptor antagonist for the ultra-rare genetic disease, WHIM syndrome. WHIM is short for warts, hypogammaglobulinemia, immunodeficiency, and myelokathexis, reflecting the clinical manifestations of the disease that leads to immune system dysfunction and increased infections. Low levels of circulating antibodies (hypogammaglobulinemia) and low levels of circulating neutrophil (myelokathexis) contribute to the immunodeficient state.
Xolremdi is the first therapy specifically designed for WHIM syndrome. The FDA approved Xolremdi in April this year. Previously, the FDA had granted Breakthrough Therapy Designation and Priority Review to Xolremdi for WHIM syndrome, emphasizing the unmet need for an ultra-rare disease. With Xolremdi’s approval, X4 Pharmaceuticals also received a Rare Pediatric Disease Priority Review Voucher, which can be used to expedite the review of a future application or sold to another drug sponsor. X4 plans to seek approval from the European Medicines Agency (EMA) for Xolremdi in late 2024 or early 2025.
Xolremdi is an oral drug with an annual price of $496,400 for patients weighing over 50 kg (400 mg daily) and $372,300 for patients weighing 50 kg or less (300 mg daily, assuming full patient compliance).
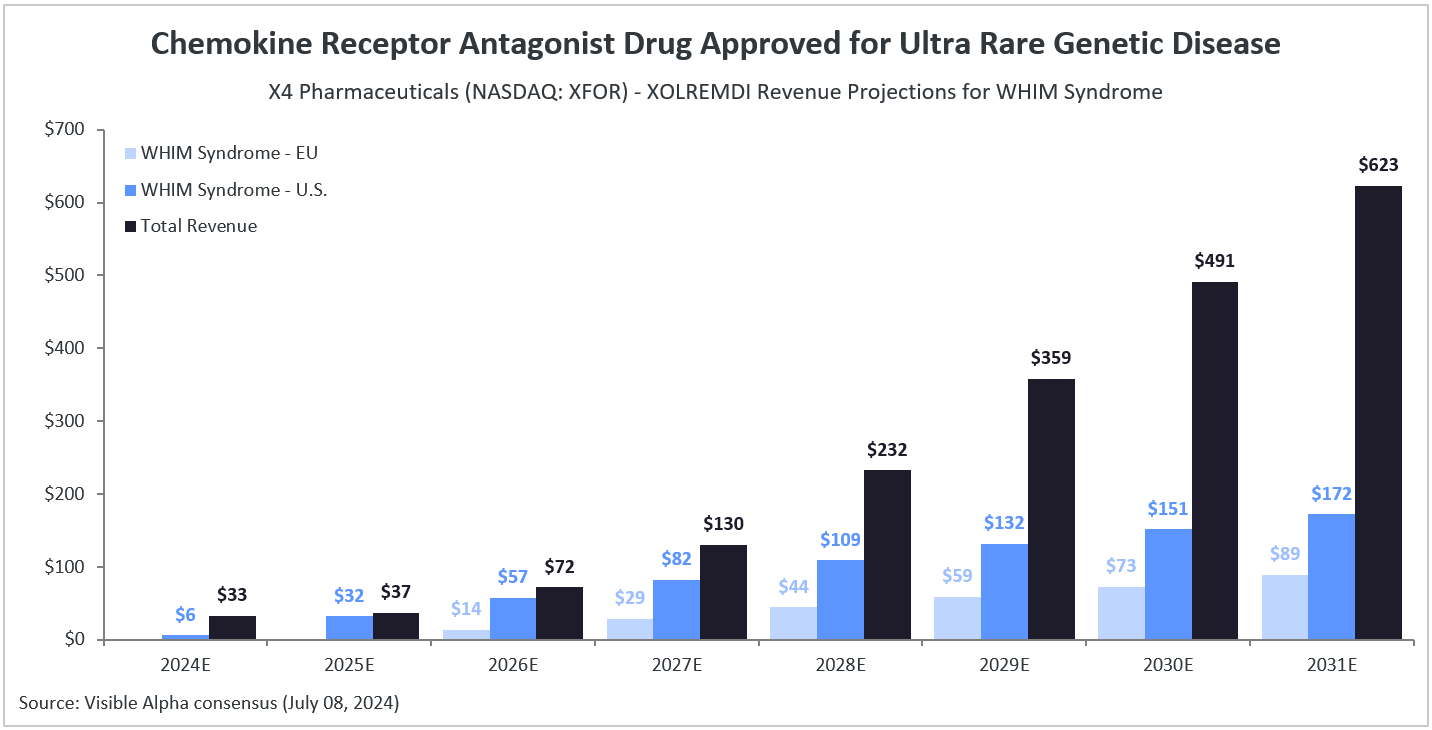
According to Visible Alpha consensus estimates, Xolremdi is expected to generate $6 million in U.S. revenue in 2024, with U.S. revenues increasing to $32 million in 2025 and $172 million by 2031. Following potential approvals in the EU, global sales are projected to reach $261 million by 2031. The Xolremdi patent is set to expire on December 31, 2027. In 2024, XOLREMDI for WHIM syndrome is estimated to account for 19% of the company’s overall revenue, with revenue share increasing to 91% by 2025.
More about WHIM syndrome and the CXCR4 chemokine receptor
An estimated 1000 individuals in the U.S. have the ultra-rare disease WHIM syndrome. WHIM syndrome is caused by genetic mutations in the CXCR4 gene which codes for the CXCR4 chemokine receptor found on stem cells and immune cells. CXCR4 binds to its ligand CXCL12 and induces cell signaling that orchestrates cell migration, hematopoiesis and cell homing, and retention of immune cells in the bone marrow. Mutations in the CXCR4 gene, as in WHIM syndrome, can lead to a “gain of function” that increases CXCR4 receptor activity and prevents mature neutrophils from leaving the bone marrow and entering circulation in the blood. By blocking the CXCR4 receptor, as with Xolremdi, the “gain of function” effect can be blunted. There are other drugs approved that are CXCR4 antagonists. An example is plerixafor, as a hematopoietic stem cell mobilizer for autologous stem transplantation in certain leukemias. Plerixafor is marketed by Sanofi (NASDAQ: SNY). CXCR4 also has a major role in cancer.
Reference:
https://investors.x4pharma.com/news-releases/news-release-details/x4-pharmaceuticals-announces-fda-approval-xolremditm-mavorixafor