Eli Lilly’s (NYSE: LLY) donanemab treatment for Alzheimer’s disease has been subject to extended regulatory delays. Analysts and investors had expected that the FDA would approve donanemab in Q1 2024. In early March, however, the FDA announced that it would convene an Advisory Committee meeting to further evaluate the donanemab approval application. While it is not uncommon to convene an Advisory Committee meeting, it is unusual to convene it so late in the process.
The FDA communicated to Eli Lilly that the Advisory Committee meeting will further evaluate the safety and efficacy of donanemab in the Phase 3 trial. In particular, the FDA needs to understand the implications of the unique Phase 3 trial design that included limited-duration dosing, which allowed completion of treatment based on measure of amyloid plaque. In addition, the trial was also unique in using tau protein levels as an inclusion criterion.
Donanemab’s regulatory history
The company had announced the completion of Phase 3 trials (TRAILBLAZER-ALZ 2 study) and subsequent submission of the donanemab Biologics License Application (BLA) in July 2023. It was expected that donanemab would be approved by the end of 2023. In November 2023, however, the FDA requested additional data from Eli Lilly, pushing approval to Q1 2024. Currently, with FDA plans for an Advisory Committee meeting, donanemab approval has been pushed further to Q2 2024.
Prior to completion of the Phase 3 trial in July 2023, the FDA had declined in January 2023 to grant donanemab Accelerated Approval. The FDA was likely seeking more clarity on safety and efficacy after completion of the larger Phase 3 trial.
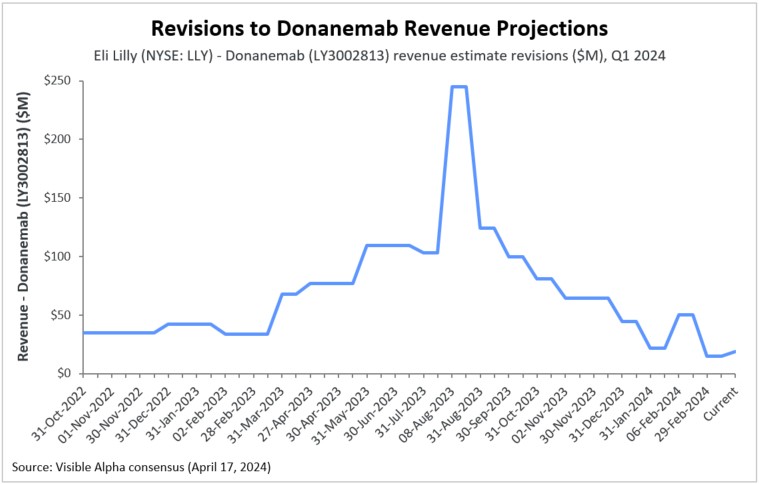
Revisions to donanemab revenue projections
Given the regulatory delays and stiff competition from Biogen’s (NASDAQ: BIIB) and Eisai’s (TSE: 4523) Leqembi, which was approved for early-stage Alzheimer’s disease in the summer of 2023, Visible Alpha consensus shows analysts have revised donanemab revenue projections downward. (See also our previous article on Novel Therapies for Treating Alzheimer’s Disease.)
Figure 1: Revisions to donanemab revenue projections
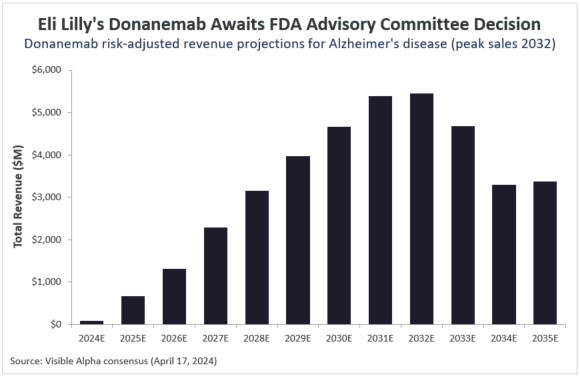
Based on Visible Alpha consensus estimates, donanemab is expected to reach risk-adjusted peak sales of $5.4 billion in 2032. Furthermore, the likelihood of approval is pegged at 91.7%, implying that analysts continue to believe that donanemab will likely be approved by the FDA, even though there is additional regulatory scrutiny.