Clinical researchers across the globe, including from Novo Nordisk (NYSE: NVO), recently published a long-term study evaluating the company’s obesity drug, Wegovy (a semaglutide), in patients that had pre-existing cardiovascular disease and were overweight or obese, but were not diabetic. The results of this trial validate the safety, efficacy, and durability of Wegovy in obesity/weight management and cardiovascular benefit. Wegovy may be the first weight-loss drug that has such impressive and durable cardiovascular benefits. (See also our Visible Alpha GLP-1 Monitor for more on this family of drugs.)
This trial was termed “Semaglutide Effects on Cardiovascular Outcomes in People with Overweight or Obesity,” or the SELECT trial. It enrolled 17,604 patients across 41 countries treated with Wegovy or placebo (control) that were evaluated over four years. The SELECT trial marks the longest clinical trial that evaluates the effects of a semaglutide (once-weekly Wegovy injection) versus placebo on weight loss, safety, and durability in a geographically and racially diverse population that is overweight or obese, but not diabetic.
Summary of SELECT trial results
The primary endpoint of the study was the time to first occurrence of a composite endpoint consisting of: cardiovascular (CV) death, non-fatal myocardial infarction, or non-fatal stroke within a 59-month time frame. Secondary endpoints included several measures within a 59-month timeframe related to cardiovascular metrics (including cardiovascular death and all causes of death) and several endpoints related to blood pressure, blood metabolism chemistry, and change in body weight and waist circumference measured at baseline and at 2 years. Body Mass Index (BMI) was used to measure weight loss over the four years of the study. BMI is a measure of body fat based on height and weight.
The results of the SELECT trial can be summarized as follows for 2.4 mg Wegovy administration every week over 208 weeks (almost 48 months or four years) versus placebo:
- Mean reduction in weight of 10.2% in the Wegovy arm versus 1.5% in the placebo arm
- Waist circumference reduction of 7.7 cm in the Wegovy arm versus 1.3 cm in the placebo arm
- Waist-to-height ratio reduction of 6.9% in the Wegovy arm versus 1.0% in the placebo arm
- P < 0.0001 for all the above comparisons of Wegovy-treated versus placebo-treated arm
- Weight loss was sustained over the four years while on Wegovy
The individuals in the trial were grouped in four categories based on BMI (<30, 30 to <35, 35 to <40, and ≥40 kg/m2). Each of the BMI categories had lower rates of serious adverse events in the Wegovy-treated arm versus placebo arm, pointing to the safety and tolerability of Wegovy. Notably, the difference in the rate of serious adverse events between the Wegovy-treated and placebo arm was most pronounced in the highest BMI group – the more obese individuals had increased serious adverse events in the placebo arm compared to the Wegovy arm. This demonstrates the significant efficacy and safety benefit of Wegovy treatment compared to placebo.
Figure 1: Wegovy vs. placebo for weight loss
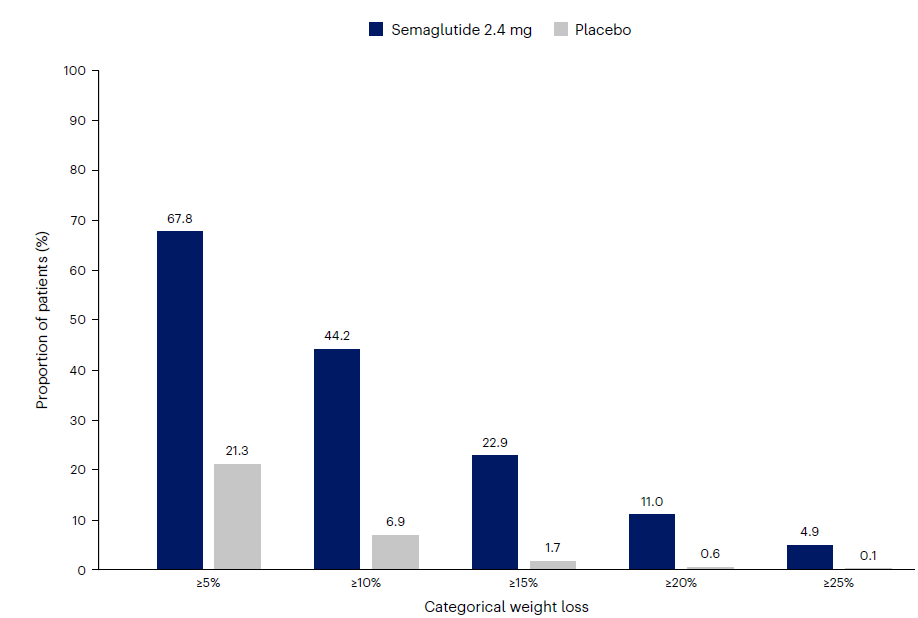
Source: Ryan et al; Nature Medicine. May 13, 2024. Categorical weight loss from baseline at week 104 for Wegovy and placebo in the SELECT trial. Data is from the in-trial period. Bars depict the proportion (%) of patients receiving Wegovy or placebo who achieved ≥5%, ≥10%, ≥15%, ≥20%, and ≥25% weight loss.
The results of this trial, especially on cardiovascular safety metrics, are likely to expand the market potential of Wegovy and the other major semaglutide in Novo Nordisk’s portfolio, Ozempic. Ozempic is approved for type 2 diabetes, not for obesity/weight management. Note that in March 2024 the FDA expanded Wegovy approval for cardiovascular benefit in addition to Wegovy’s initial approval for obesity/weight management.
According to Visible Alpha consensus, analysts’ revenue projections estimate Wegovy will reach $9 billion in 2024 and top $22.5 billion in 2030.
Figure 2: Novo Nordisk’s Wegovy consensus revenue projections
Reference:
- Ryan et al; Nature Medicine; Long-term weight loss effects of semaglutide in obesity without diabetes in the SELECT trial. May 13, 2024




