Merck’s (NYSE: MRK) MK-0616 is an oral PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor drug candidate currently in Phase 3 studies for hypercholesterolemia. This drug could be the first oral PCSK9 inhibitor that makes it to market, which could significantly change the landscape for hypercholesterolemia treatment.
PCSK9 inhibition is an effective therapeutic tool, usually as an add-on to statins, in the control of hypercholesterolemia that is not controlled by statins alone. Currently approved PCSK9 inhibitors are administered by injection (antibodies or siRNA-based drugs). The prospect of an oral PCSK9 inhibitor may have the potential to expand the current PCSK9 inhibitor market.
Currently marketed PCSK9 inhibitors have fallen short of initial market expectations
PCSK9 inhibitors were projected to be blockbuster drugs when first approved. The currently approved PCSK9 inhibitors are Amgen’s (NASDAQ: AMGN) Repatha, Sanofi (NASDAQ: SNY) and Regeneron’s (NASDAQ: REGN) Praluent, and Alnylam (NASDAQ: ALNY) and Novartis’ (NYSE: NVS) Leqvio. Repatha and Praluent are monoclonal antibodies, and Leqvio is RNA-interference based. All three are subcutaneous injections. The clinical experience with PCSK9 inhibitors had shown effective LDL-cholesterol lowering across most patient subgroups, along with safety. In patients with atherosclerotic cardiovascular disease (ASCVD) where more aggressive LDL-cholesterol reduction is required, PCSK9 inhibitors in combination with statins may be the most effective in reaching treatment goals of lowering LDL-cholesterol.
Figure 1: PCSK9 inhibitors on the market
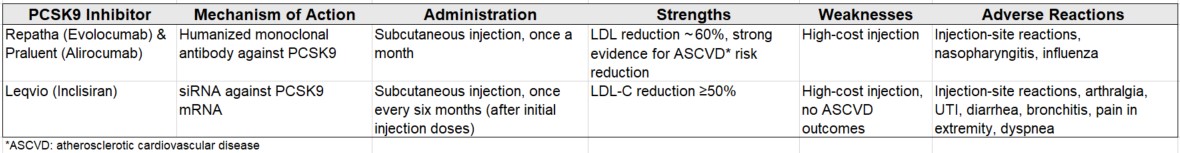
Adapted from Agnello et al; J. Clin. Med. 2024, 13, 125. Repatha (MRK) and Praluent (SNY & REGN) are monoclonal antibodies against PCSK9. Leqvio (ALNY and NVS) is RNA interference/siRNA targeted against PCSK9 mRNA. All three approved PCSK9 inhibitors are injections.
However, market acceptance has been less than expected since the approval of Repatha and Praluent in 2015, and then Leqvio in 2020. This lower-than-expected market success of PCSK9 inhibitors can be attributed to the high cost, a complex insurance approval process, and the fact that the approved PCSK9 inhibitors have all been injections. Importantly, patients are reluctant to self-inject or choose injections as an add-on to statins for a condition that does not have symptoms. Patient compliance was also a factor — patients on statins often did not comply with monthly injections after the first few injections. Compared to Repatha and Praluent monthly injections, Leqvio has the advantage of twice-yearly injections after the first year.
In 2018-19 the cost of Repatha and Praluent was reduced significantly by about 60%, bringing the cost down to approximately $5,800 for annual therapy. The cost of Leqvio is $9,700 for the first year and then $6,700 for subsequent years.
Analysts project improved prospects for Repatha & Leqvio
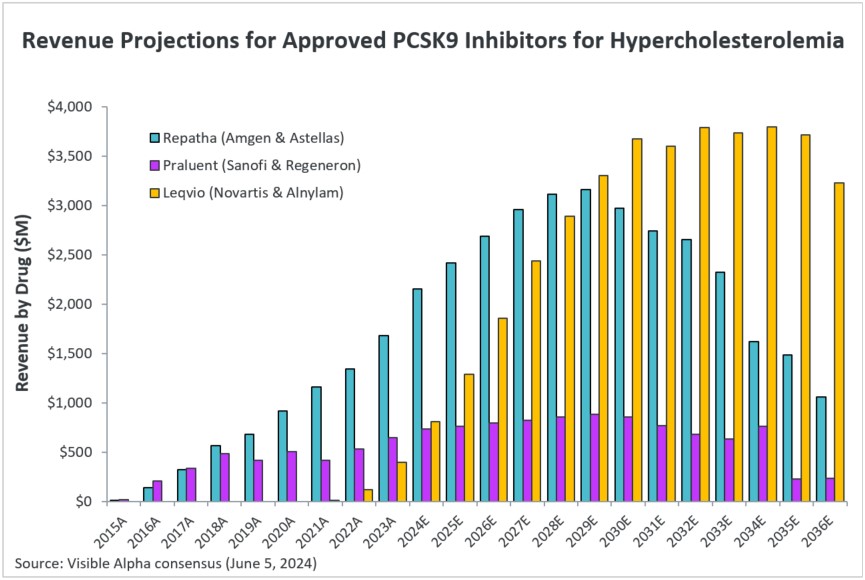
Visible Alpha consensus shows increasing estimated revenues in the coming years for Repatha and Leqvio, potentially as a result of a reduction in cost that is far more acceptable for insurance. Praluent revenue projections are modest. For example, in 2023, Repatha revenues were $1.7 billion — based on Visible Alpha consensus, Repatha revenues in 2024 are estimated at $2.6 billion, and reach $3.1 billion in 2028. Leqvio revenues in 2023 were $396 million and are estimated at $808 million in 2024, reaching $3.3 billion in 2029. Leqvio is a siRNA-based injection that requires a less frequent dosing schedule compared to the monoclonal antibodies, Repatha and Praluent. Efficacy in LDL-cholesterol reduction is generally considered similar between the monoclonal antibody-based PCSK9 inhibitors (Repatha/Praluent) and siRNA based Leqvio. However, unlike for Repatha and Praluent, it should be noted that there is no cardiovascular outcomes study with Leqvio showing reduction in risk of atherosclerotic cardiovascular disease (ASCVD).
Figure 2: Consensus revenue projections for currently approved PCSK9 inhibitors
MK-0616 could be the first approved oral PCSK9 inhibitor
The development of an oral PCSK9 inhibitor is a significant achievement since PCSK9 interaction with the LDL-receptor does not easily cater to small molecule drug inhibition. Merck’s MK-0616 is an oral macrocyclic peptide that has demonstrated efficacy and safety in Phase 2b studies.
Phase 2b clinical trial data with MK-0616
Merck conducted a Phase 2b, randomized, double-blind, placebo-controlled, multicenter trial to evaluate the efficacy and safety of MK-0616 in patients with hypercholesterolemia. A total of 381 patients were enrolled. The doses of MK-0616 used were 6, 12, 18, or 30 mg administered once daily, or placebo. The reduction in LDL-cholesterol from baseline to 8 weeks vs placebo was 41.2% (6 mg), -55.7% (12 mg), -59.1% (18 mg), and -60.9% (30 mg). All doses of MK-0616 were statistically significant. MK-0616 was well tolerated as adverse events were comparable between treatment arms and placebo. A reduction of LDL-cholesterol from baseline of 60.9% in the highest dose (30 mg) is impressive.
Comprehensive Phase 3 clinical program initiated with MK-0616
Merck has initiated a comprehensive Phase 3 program that also includes a cardiovascular outcomes study, besides a lipid-lowering study and a supportive study in different hypercholesterolemia subgroups. A total of 17,000 patients will be enrolled across the three trials:
- A Study of MK-0616 (Oral PCSK9 Inhibitor) in Adults With Hypercholesterolemia (MK-0616-013) CORALreef Lipids (NCT05952856 https://classic.clinicaltrials.gov/ct2/show/NCT05952856)
- A Study of MK-0616 (Oral PCSK9 Inhibitor) in Adults With Heterozygous Familial Hypercholesterolemia (MK-0616-017) CORALreef HeFH (NCT05952869 https://classic.clinicaltrials.gov/ct2/show/NCT05952869)
- MK-0616 (Oral PCSK9 Inhibitor) Cardiovascular Outcomes Study (MK-0616-015) CORALreef Outcomes (NCT06008756 https://classic.clinicaltrials.gov/ct2/show/NCT06008756)
MK-0616 revenue potential
According to Visible Alpha consensus, analysts project that MK-0616 will be on the market in 2026 and reach an estimated $4.8 billion in revenues by 2035. Risk-adjusted revenues in 2035 are an estimated $2.9 billion. Visible Alpha consensus probability of success for MK-0616 approval is 67.4%. If approved, MK-0616 will be the first oral PCSK9 inhibitor on the market. An oral PCSK9 inhibitor is expected to have advantages over the currently marketed PCSK9 inhibitors that are all injections.
Figure 3: Consensus revenue projections for Merck’s MK-0616
AstraZeneca’s oral PCSK9 inhibitor follows in the footsteps of MK-0616
AstraZeneca (NASDAQ: AZN) is developing an oral small molecule PCSK9 inhibitor, AZD0780. Phase 1 studies have been completed. A statistically significant reduction of 52% in LDL-cholesterol levels was observed when AZD0780 was added to statin treatment (78% reduction from baseline) in treatment-naive participants with hypercholesterolemia. The company has initiated Phase 2 studies with AZD0780.
Besides the oral PCSK9 inhibitors in development, other approaches targeting PCSK9 include gene silencing, DNA base editing, and vaccine therapies. These are in earlier stages of development and are not discussed in this report.
About PCSK9 and hypercholesterolemia
PCSK9 is involved in cholesterol homeostasis and is related to the concentration of cholesterol in the cell. Elevated levels of circulating PCSK9 are associated with increased low-density lipoprotein cholesterol (LDL-cholesterol) and worse cardiovascular outcomes.
PCSK9, an enzyme (serine protease) encoded by the PCSK9 gene, is predominantly produced in the liver. PCSK9 binds to the LDL-receptor on the surface of hepatocytes, leading to the degradation of the LDL-receptor and subsequently to higher plasma LDL-cholesterol levels. When PCSK9 is inhibited and not attached to the LDL-receptor, the LDL-receptor is recycled back to the cell surface and can reduce serum LDL-cholesterol (see Figure 4, below). PCSK9 inhibitors are usually given as an add-on to statin treatment.
Figure 4: Mechanism of action of PCSK9 inhibitors
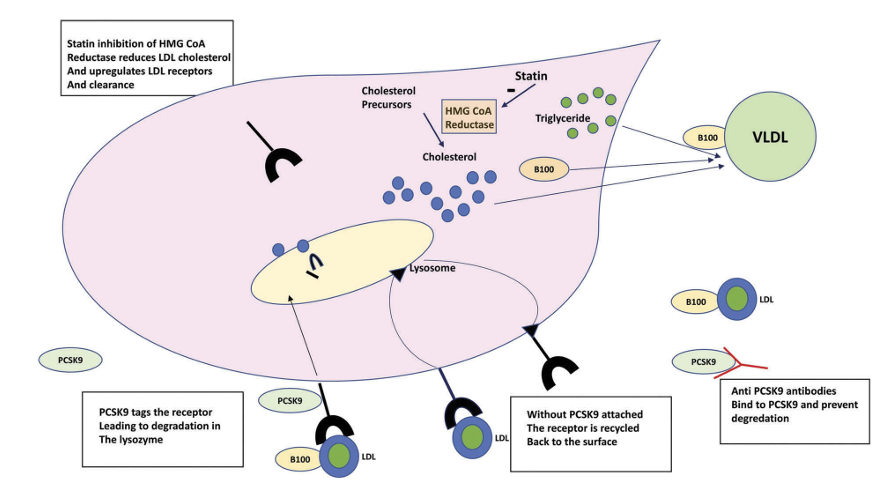
Source: Fitzgerald & Kiernan; 2018; Expert Review of Cardiovascular Therapy, 16:8, 567-578. PCSK9 regulates LDL-receptors (LDL-R) and its inhibition increases LDL-R recycling to the cell surface of hepatocytes and subsequent lowering of LDL-cholesterol (LDL-C) concentration in serum.
References:
- Chapman & Packard; Realizing the Potential of PCSK9 Inhibition: A Novel Oral Macrocyclic Peptide on the Horizon; Journal of the American College of Cardiology. 2023, 81 (16) 1565–1568.
- Agnello F et al; Novel and Emerging LDL-C Lowering Strategies: A New Era of Dyslipidemia Management. Journal of Clinical Medicine. 2024, 13, 1251.
- Ballantyne MC et al; Phase 2b Randomized Trial of the Oral PCSK9 Inhibitor MK-0616; Journal of the American College of Cardiology. 2023, 81:16, 1553-1564.
- Gerald Fitzgerald & Tom Kiernan; PCSK9 inhibitors and LDL reduction: pharmacology, clinical implications, and future perspectives. Expert Review of Cardiovascular Therapy. 2018, 16:8, 567-578.
- Dayoub EJ et al; Adoption of PCSK9 Inhibitors Among Patients With Atherosclerotic Disease. Journal of the American Heart Association. 2021,10 (9).