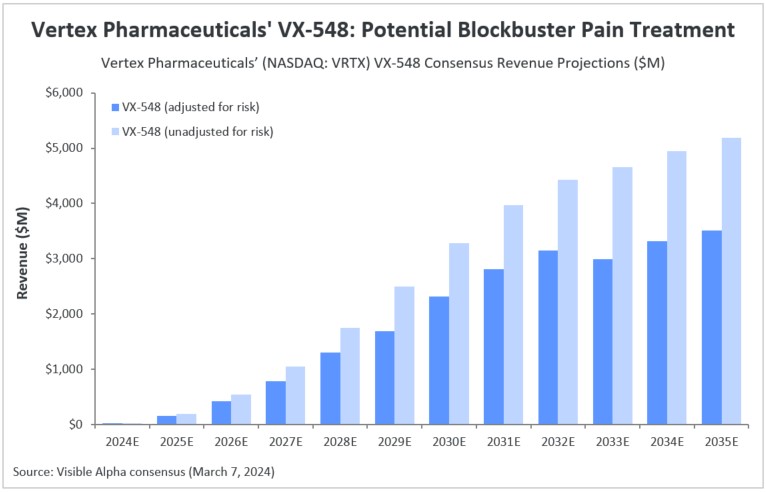
Vertex Pharmaceuticals (NASDAQ: VRTX) is expected to generate $21.1 million in revenue from its non-opioid pain treatment VX-548 in 2024. The company is currently preparing a new drug application (NDA) for VX-548, which has been granted both breakthrough therapy and fast track designations. This application is set to be submitted to the FDA by mid-2024. According to Visible Alpha consensus, analysts project risk-adjusted revenue of $1.3 billion by 2028 and $3.3 billion by 2034. Analysts estimate the likelihood of success for VX-548 at 75%.
About VX-548
VX-548’s mechanism does not hold addictive potential, thereby achieving opioid-like efficacy without the abuse potential of that class of effective, but problematic, drugs. In January, Vertex announced successful results from three Phase 3 studies of VX-548, showing significant pain reduction for both surgical and non-surgical patients. However, it failed to meet the secondary goal in two trials of reducing pain when compared to a combination of the opioid drug hydrocodone and acetaminophen, the basis for popular pain medications like Tylenol. Vertex is also pursuing a broad label for VX-548 in peripheral neuropathic pain, with positive Phase 2 results in painful diabetic peripheral neuropathy reported and plans to advance pivotal development in this area.
Prior to VX-548, Vertex discontinued development of three other non-opioid drugs—VX-128, VX-150, and VX-961—due to unsuccessful Phase 1 and 2 trials. This makes VX-548’s Phase 3 results a significant win for Vertex.
Potential advantages of VX-548
Selective inhibition of voltage-gated sodium channel NaV1.8 with VX-548 for acute pain is a novel approach. Most approved pain drugs either act on the opioid-receptor system, or are non-steroidal anti-inflammatory drugs (NSAIDs), or are non-selective sodium-channel inhibitors (for example, lidocaine). Opioid use is limited by safety concerns and the potential for misuse and addiction, and NSAIDS and non-selective sodium-channel inhibitors have safety and efficacy limitations. Selective inhibition of voltage-gated sodium channel NaV1.8 is an important addition in the armory of pain drugs.
Reviewed by Rahul Jasuja, PhD