In June, Abbott Laboratories (NYSE: ABT) obtained FDA approval for two new continuous glucose monitoring (CGM) systems available over the counter: Lingo and Libre Rio. Lingo targets individuals without diabetes aiming to enhance their metabolic health, while Libre Rio is designed for adults with Type 2 diabetes not using insulin to manage their condition. Both devices can be linked to a smartphone app and use Abbott’s FreeStyle Libre technology, a medical device that monitors glucose spikes. Libre Rio is Abbott’s first over-the-counter CGM system and will join its overall Libre portfolio of CGM systems, which consists of the FreeStyle Libre 2 and FreeStyle Libre 3 systems for people with all types of diabetes.
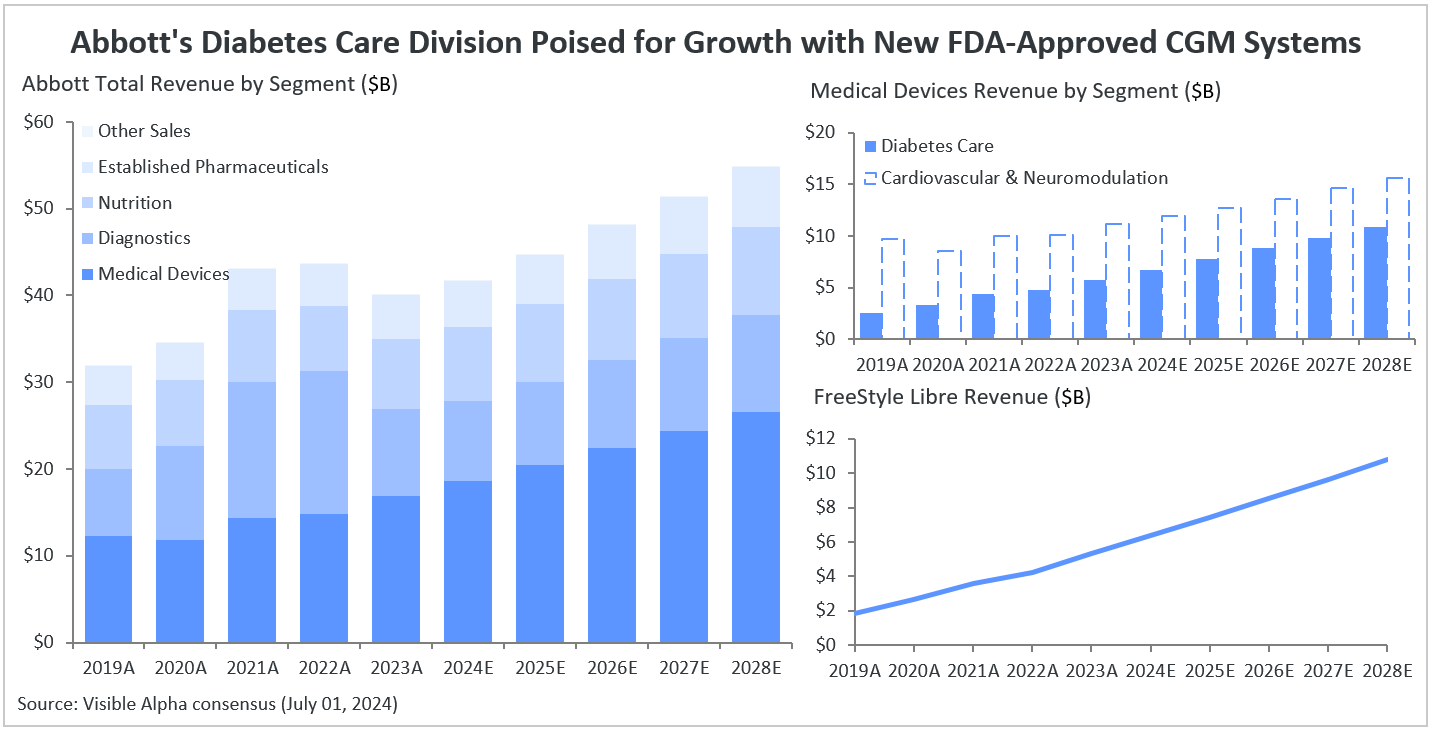
Abbott operates across four primary business segments: medical devices, diagnostics, nutrition, established pharmaceuticals, and others. Medical devices have been a significant revenue driver, constituting about 45% of the company’s total revenue in 2024. Analyst projections indicate this share is anticipated to increase to 51% by 2030. Within the medical devices segment, diabetes care, and particularly sales from the FreeStyle device, plays a crucial role.
According to Visible Alpha consensus, FreeStyle Libre is estimated to contribute approximately 15% ($6.4 billion) to Abbott’s total revenue in 2024, with expectations for this share to rise to 21% by 2030. Overall, Abbott is forecasted to experience a revenue rebound in 2024, following a challenging 2023 marked by a -8% decline in revenues year over year, primarily due to reduced sales related to COVID-19 testing. Visible Alpha consensus projects +4% revenue growth to $41.7 billion for Abbott in 2024.