On April 3, 2024, Danish biotech company Genmab (NASDAQ: GMAB) agreed to acquire ProfoundBio for $1.8 billion. With this acquisition, Genmab joins a growing list of companies developing antibody-drug conjugate (ADC) based therapies. The acquisition was driven by three clinical-stage programs, including the most mature program, Rina-S (rinatabart sesutecan), currently in a Phase 2 trial for ovarian cancer and other solid tumors.
Rina-S is a novel, next-generation, potentially best-in-class ADC that targets folate receptor alpha (FRα) in development for treating ovarian cancer and other solid tumors. The cytotoxic payload attached to the FRα antibody is a topoisomerase 1 inhibitor, exatecan. The FRα is expressed at high levels on several tumor types and has limited expression on normal tissue, making it an attractive target for antibody-based approaches. The FDA has granted Rina-S Fast Track designation for the treatment of patients with FRα-expressing high-grade serous or endometrioid platinum-resistant ovarian cancer.
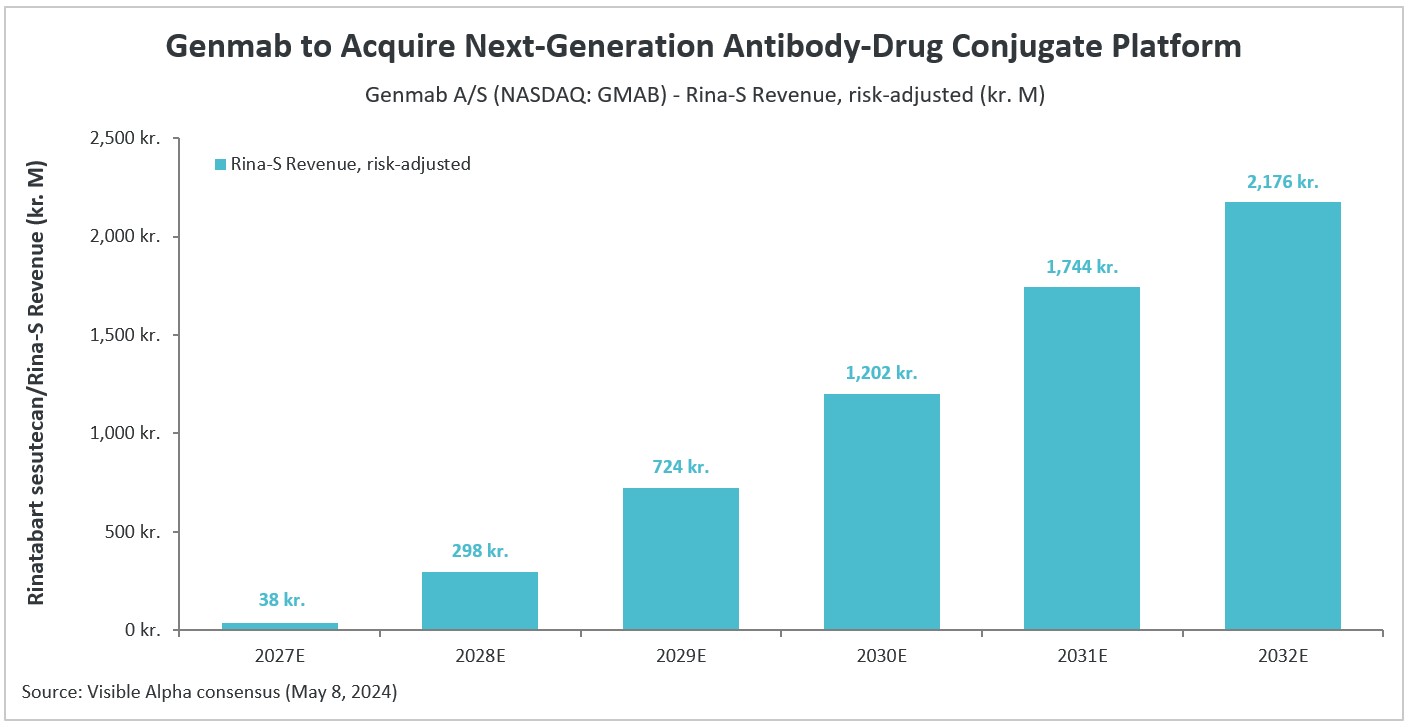
As a potential best-in-class ADC, Rina-S may address a broader patient population than first-generation FRα-targeted ADCs (like AbbVie’s Elahere). Besides AbbVie (ABBV), competition in the ADC field includes Pfizer (PFE), AstraZeneca (AZN), and Merck (MRK). Based on Visible Alpha consensus, Rina-S is projected to be on the market in 2027 and is estimated to generate approximately kr.2.2 billion ($317 million) in risk-adjusted revenues by 2032. Analysts peg the probability of success (POS) for Rina-S approval at around 45%.