Gritstone Bio (NASDAQ: GRTS) is developing a next-generation personalized therapeutic cancer vaccine based on its tumor-specific neoantigen platform (GRANITE). Neoantigens are tumor antigens unique to the patient and/or the tumor and are not expressed on normal cells. The GRANITE program has received the FDA’s Fast Track designation and is currently in Phase 2/3 trials as a maintenance therapy in patients with newly diagnosed metastatic microsatellite-stable colorectal cancer (MSS-CRC). Results from the Phase 2 portion of the Phase 2/3 GRANITE trial missed its primary endpoint measuring circulating tumor DNA (ctDNA). A secondary endpoint of progression-free survival had encouraging trends but was not statistically significant.
Analysts see increased risk following Phase 2 data
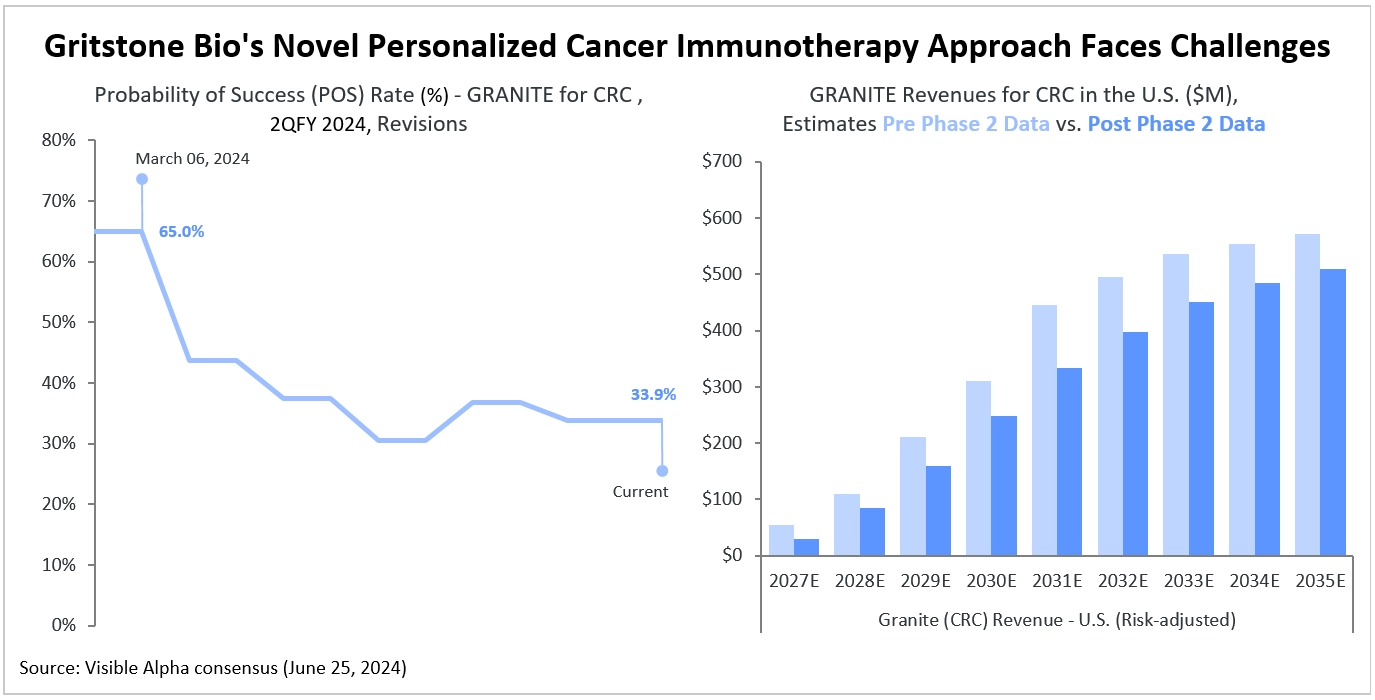
Visible Alpha consensus shows that following the Phase 2 GRANITE data release, analysts perceive heightened risk in the program, with the consensus probability of success (POS) for FDA approval dropping to 33.9% from the 65% POS expected in March. Based on Visible Alpha consensus, analysts expect GRANITE for colorectal cancer (CRC) to generate $29 million in risk-adjusted revenues in 2027 in the U.S. markets. By 2035, risk-adjusted revenues in the U.S. are projected to reach $509 million.
Phase 2 portion of Phase 2/3 GRANITE study misses primary endpoint
The Phase 2 portion of the Phase 2/3 study failed to meet its primary endpoint of a short-term molecular response defined as at least a 30% decline from baseline in circulating tumor DNA (ctDNA). The data showed that only 30% of patients who received GRANITE achieved this molecular response, compared to 42% in the control group.
Gritstone attributed these results to a misunderstanding of how ctDNA would change after treatment – ctDNA decreased for longer than expected on chemotherapy in both arms, resulting in similar molecular response in the short term. The longer-term evaluation shows the expected correlation between ctDNA with clinical benefit and favors GRANITE patients. Progression-free survival (PFS) trends were encouraging but not statistically significant. Gritstone reported hazard rates (HR) of 0.82 for the overall population and 0.52 for a subgroup of high-risk patients with increased risk of disease progression – the vast majority of this group have liver metastases. The data was more mature in the high-risk group and the clinical effect of GRANITE was pronounced in this high-risk group.
Longer-term PFS data is expected in 3Q 2024. The company chose to use ctDNA, an untested measure, due to concerns that immunotherapies can sometimes cause tumors to enlarge temporarily as they infiltrate the cancer (pseudo-progression).
About Gritstone’s personalized neoantigen vaccine approach
A salient feature of a neoantigen vaccine approach is the potential to induce a potent cytotoxic CD8+ T cell-driven anti-tumor immune response that is personalized to the patient’s unique tumor antigens (neoantigens). Tumor antigen heterogeneity within tumors and from patient to patient is a major challenge in cancer immunotherapy, and the GRANITE personalized neoantigen approach has the potential to address this unmet need.
Neoantigens are peptides found exclusively within cancer cells and absent in normal cells. A neoantigen-based therapeutic cancer vaccine approach that activates neoantigen-specific CD8 + T cells would complement checkpoint inhibitors (for example, Merck’s Keytruda or Bristol-Myers Squibb’s Opdivo) that are effective in activating pre-existing CD8+ T cells targeted against a distinct repertoire of tumor antigens that are immunogenic. This approach increases the repertoire of cytotoxic CD8+ T cells that may lead to a more potent and broad immune response.