Vertex Pharmaceuticals (NASDAQ: VRTX) has a leading position in the cystic fibrosis (CF) market, driven by Trikafta. Positive Phase 3 data for the company’s pipeline drug, vanza, was reported in February this year, and it’s likely to follow in the footsteps of Trikafta, which was approved in 2019.
Vertex conducted two Phase 3 studies with vanza (vanzacaftor/tezacaftor/deutivacaftor) that met their primary and key secondary endpoints in treating CF patients, demonstrating non-inferiority to Trikafta in enhancing lung function (measured as percent predicted forced expiratory volume in 1 second (ppFEV1)). Vanza demonstrated superiority over Trikafta in reducing sweat chloride (SwCl) levels, a secondary endpoint in the trial. Based on these results, Vertex intends to file for approval in the U.S. and Europe by mid-2024 for patients with CF ages 6 years and older. Vertex also plans to use its FDA priority review voucher, which guarantees an expedited six-month review process in the U.S.
Vanza and Trikafta are both triple-combination CFTR (cystic fibrosis transmembrane conductance regulator) protein modulators. Vanza is a next-generation CFTR modulator administered once daily, while Trikafta is administered twice daily.
Vanza may not have a clear market advantage over Trikafta
A once-daily administration with vanza versus a twice-daily administration with Trikafta may not necessarily equate to greater market penetration of vanza in CF patients. This is reflected in analysts’ consensus revenue projections for Trikafta and vanza. Based on the Phase 3 trials, vanza shows equal efficacy (non-inferiority) to Trikafta in improving lung function. Vanza did show improvement over Trikafta in a measure of sweat chloride levels; however, the measure of sweat chloride levels may not lead to improvement in lung function, and sweat chloride measures are not used routinely in clinical practice. Furthermore, there is currently no long-term clinical efficacy data for vanza.
The bar set by Trikafta is high, showing effective therapeutic benefits in lung function, pulmonary exacerbations, and durability. For a subset of patients that experience side effects with Trikafta (rash, liver enzyme dysregulation, CNS issues), vanza could potentially be a preferred alternative, though full safety data for vanza has yet to be released. The positive clinical experience with Trikafta since market approval in 2019 is another barrier for clinicians to switch patients to vanza without good reason. At this stage, it is unclear if the dosing convenience of once-daily versus twice-daily compels clinicians to make the switch from Trikafta to vanza in CF patients that are well-controlled with Trikafta.
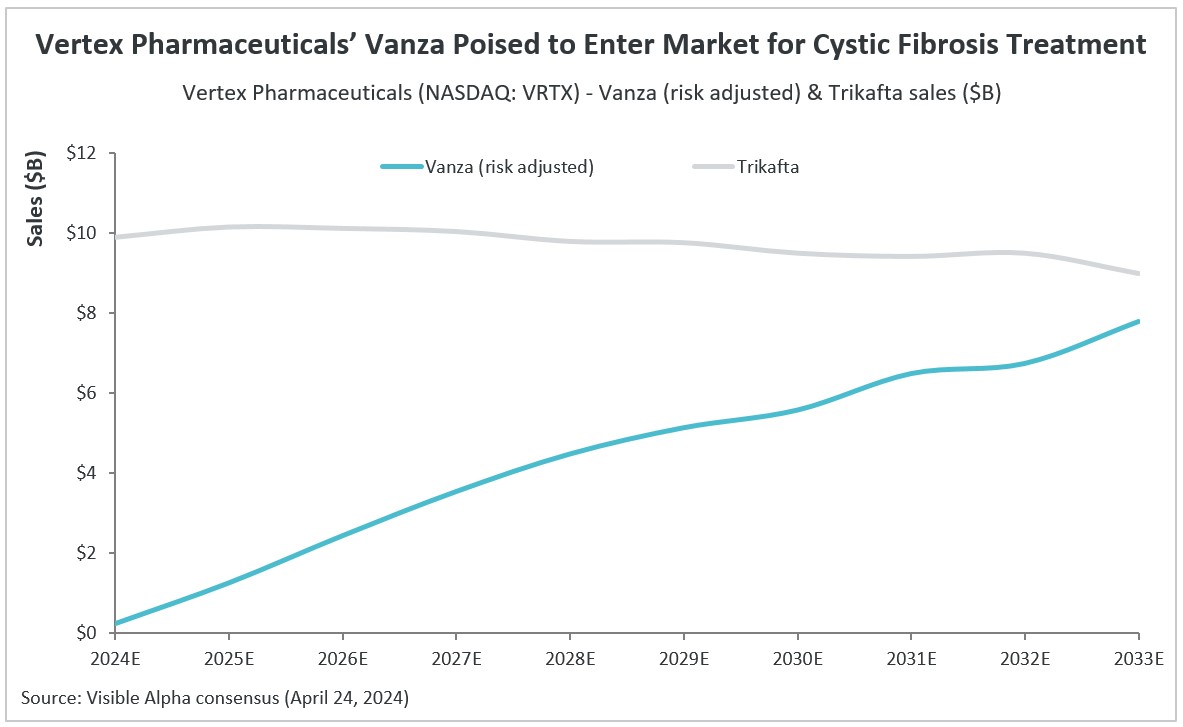
According to Visible Alpha consensus, vanza is estimated to generate $1.3 billion in risk-adjusted revenue in 2025, assuming FDA approval by the end of 2024 or early 2025. Vanza is projected to generate $7.8 billion in risk-adjusted revenues in its peak sales year of 2033. Visible Alpha consensus pegs the probability of success (POS) for vanza approval by the FDA for treating patients with CF at 90%. Vertex’s CF sales are currently driven by Trikafta, Kalydeco (ivacaftor), Orkambi (lumacaftor/ivacaftor), and Symdeko (tezacaftor/ivacaftor).
About Cystic Fibrosis and Vanza
Cystic fibrosis (CF) is a progressive, multi-organ disease caused by dysfunction or mutation of the CFTR gene. Patients with CF have a mutated protein that impacts an ion channel across the membrane of cells that produce mucus, sweat, saliva, tears, and digestive enzymes. The channel transports negatively charged chloride ions into and out of cells and controls water flow into tissues, required for generation and flow of mucus that lubricates the lining of airways and the digestive tract, among other organs. Symptoms can vary, ranging from few or no symptoms to severe or even life-threatening complications. The most serious and common complications of CF are lung-related, including frequent pulmonary or respiratory exacerbations, typically caused by serious lung infections.
Vanza is a triple combination CFTR modulator comprising two “correctors” (vanzacaftor and tezacaftor), that increase the amount of CFTR protein on the cell surface, and one “potentiator” (deutivacaftor) that increases the probability of opening the chloride ion channel. The effect of increased CFTR protein expression (i.e., increase in chloride ion channels) and increased chloride ion transport across the cell membrane provides therapeutic benefit in CF patients. Vanza was investigated in patients with the most common CF mutation in CFTR, F508del.
Reviewed by Rahul Jasuja, PhD