The FDA is slated to review Madrigal Pharmaceuticals’ (NASDAQ: MDGL) Resmetirom for patients with nonalcoholic steatohepatitis (NASH) with fibrosis — the FDA’s Prescription Drug User Fee Act (PDUFA) date is set for March 14, 2024. NASH is a dangerously progressive liver disease with no approved pharmacological agent. If Resmetirom is approved, it will be the first FDA-approved drug for NASH. Several NASH drug candidates have failed in clinical trials or in regulatory review, and a long pipeline of NASH drug candidates are in clinical trials.
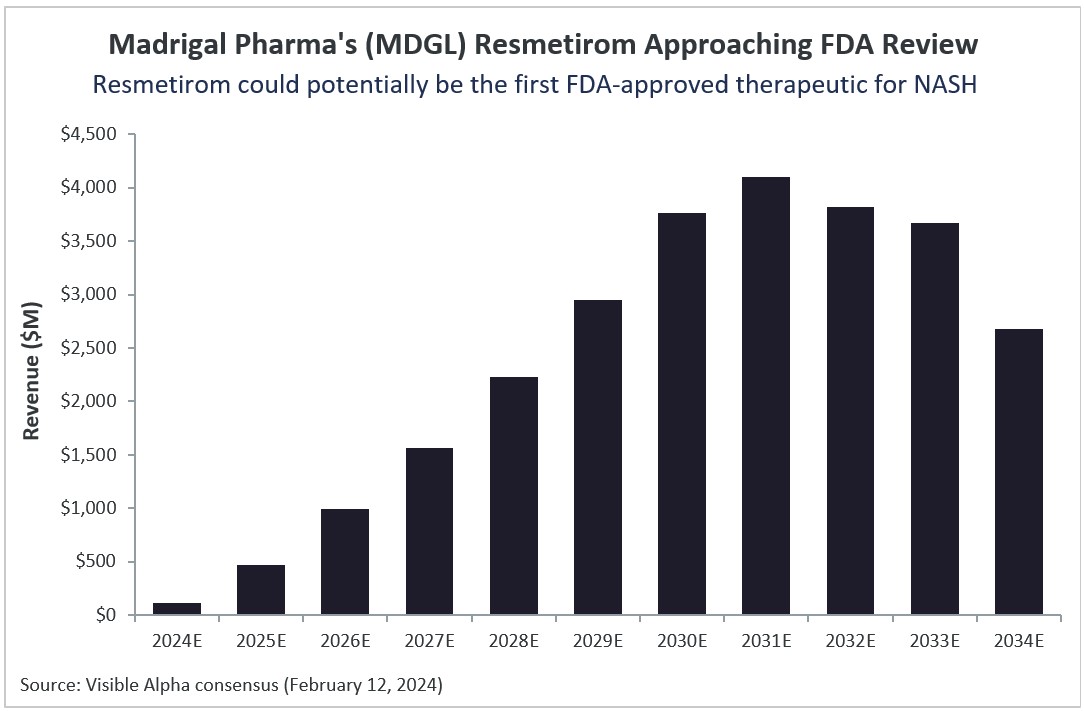
Resmetirom projections
Based on Visible Alpha consensus, Resmetirom is projected to generate over $4.1 billion in risk -adjusted revenues in its peak sales year in 2031. Visible Alpha consensus pegs the probability of success (POS) for Resmetirom approval by the FDA in NASH patients with liver fibrosis at 86.3%.
NASH and NAFLD
According to the American Liver Foundation, NASH, now also called metabolic dysfunction-associated steatohepatitis (MASH), is a form of nonalcoholic fatty liver disease (NAFLD) in which patients have inflammation of the liver and liver damage, in addition to excess fat accumulation in the liver. Patients with NASH have significant liver fibrosis leading to compromised liver function. Ultimately, NASH may progress to cirrhosis and/or liver cancer. At that stage, the liver may be permanently damaged and the only option is liver transplantation.
Resmetirom’s mechanism of action
Resmetirom has a novel mechanism of action — it is an oral thyroid hormone receptor beta-selective agonist. In NASH, the beta thyroid hormone receptor function is impaired, leading to reduction in mitochondrial function as well as reduction in β-oxidation of fatty acids that progresses to fibrosis (Harrison et al; New England Journal of Medicine 390;6, February 8, 2024). Resmetirom was a topic of discussion in our recent publication: Potential FDA Approvals: A Look Ahead for 2024.