In September 2023, Biogen (NASDAQ: BIIB) completed the acquisition of Reata Pharmaceuticals for $172.50 per share in cash, amounting to an acquisition value of about $7.3 billion. Reata Pharmaceuticals specializes in developing therapies that regulate cellular metabolism and inflammation in severe neurologic diseases. This acquisition strengthens Biogen’s neurological disease portfolio. Biogen’s acquisition was driven by Reata’s lead asset, Skyclarys (omaveloxolone), for Friedreich’s ataxia (FA) currently on the market. Skyclarys was approved in the U.S. in May 2023 and in the EU in Feb 2024.
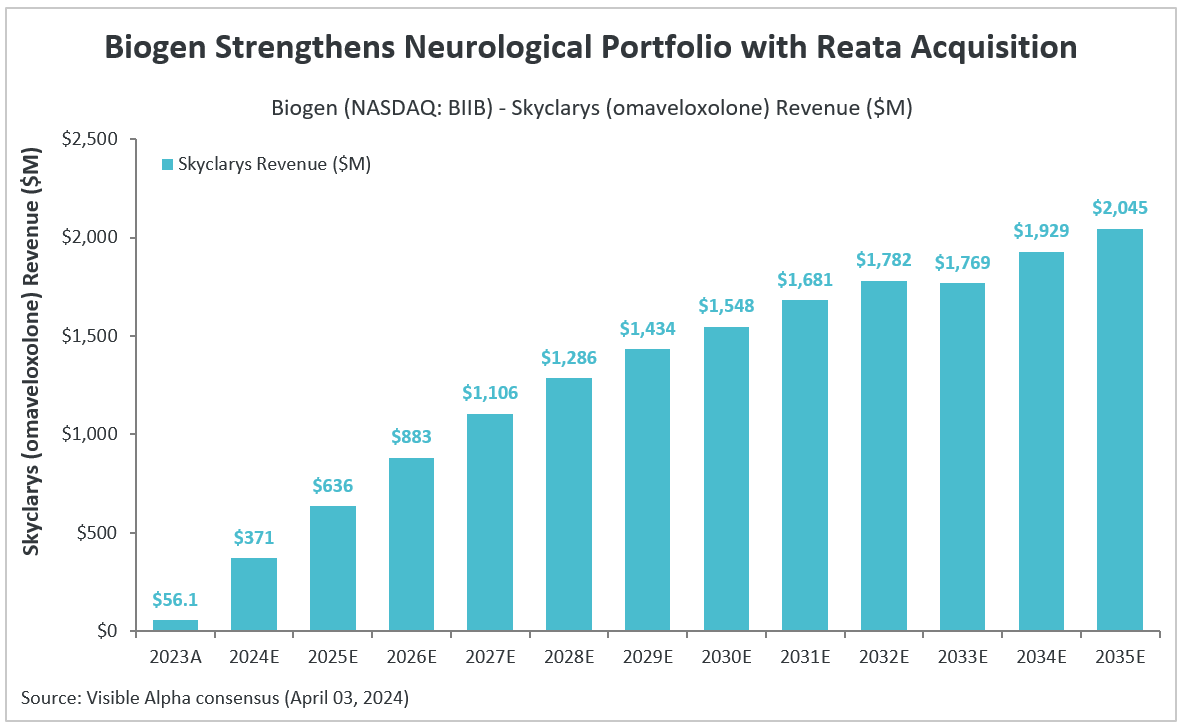
In 2023, Skyclarys generated $56 million in revenues. According to Visible Alpha consensus, Skyclarys is expected to generate $371 million in revenues in 2024 and reach peak sales of $2.2 billion by 2036.
About Friedreich’s ataxia (FA) and Skyclarys
FA is a rare, genetic disease that causes progressive damage to the nervous system, affecting approximately 5,000 patients in the U.S. FA occurs when mutations in the frataxin gene (FXN) cause mitochondrial iron accumulation leading to impaired nerve cell function. This condition damages the nervous system, resulting in muscle weakness, imbalance, and worsening of sensory deficits over time. Frequently, cardiomyopathy may also be observed. Symptoms typically start in childhood but can also begin in adulthood.
Skyclarys is the first and only FDA-approved treatment for FA (see our recent report on 2023 FDA Drug Approvals). Skyclarys activates the nuclear factor-erythroid 2-related factor 2 (NRF2) pathway, a pathway suppressed in patients with FA as a result of a mutated frataxin protein.
References
- Lynch DR, et al., Safety and Efficacy of Omaveloxolone in Friedreich Ataxia (MOXIe Study). Ann Neurol. 2021 Feb;89(2):212-225. doi: 10.1002/ana.25934
- NIH National Institute of Neurological Disorders and Stroke. https://www.ninds.nih.gov/health-information/disorders/friedreich-ataxia#toc-what-is-friedreich-ataxia-
Reviewed by Rahul Jasuja, PhD